PHOTOSENSITIZER DEVELOPMENT
We are developing new photoluminescent based on Earth-abundant elements. Current research efforts are mainly focused on early transition metals that exhibit excited states with significant ligand-to-metal charge-transfer (LMCT) character and long lifetimes due to thermally activated delayed fluorescence (TADF).
Additional research directions include molecules incorporating heavy main group elements of group 14 (Si, Ge, Sn, Pb) and lanthanides that can facilitate fast intersystem crossing to long-lived ligand-to-ligand charge-transfer (LLCT) states.

A FOCUS ON EARTH-ABUNDANT ELEMENTS
The efficient utilization of solar energy on a global scale remains a major challenge with significant implications for renewable, energy-efficient technologies and sustainable chemical processes. Most molecular photosensitizers currently used in photophysical and photochemical applications rely on rare and precious elements (shown in gray or purple on the right). This represents a significant barrier for a switch to a solar economy due to limited supply, high financial costs, and environmental impacts of resource extraction.
In the Milsmann Lab, we focus on developing new photoactive molecules based solely on Earth-abundant elements (shown in red, orange, and yellow on the right) to provide new materials that can be used in a more sustainable manner.

PHOTOSENSITIZER DESIGN PRINCIPLES FOR EARLY TRANSITION METALS
The high abundance of early transition metals makes them ideal candidates for novel photosensitizer development. However, the fundamentally different electronic structures of these elements compared to precious metals requires new approaches to the generation of photoexcited states.
We have recently shown that a combination of electron-rich ligands with electron-poor early transition metals like zirconium and hafnium provides a surprisingly simple new paradigm for the design of luminescent molecules. This approach represents a reversal of the photoinduced charge-separation step commonly encountered in late (precious) metal compounds.
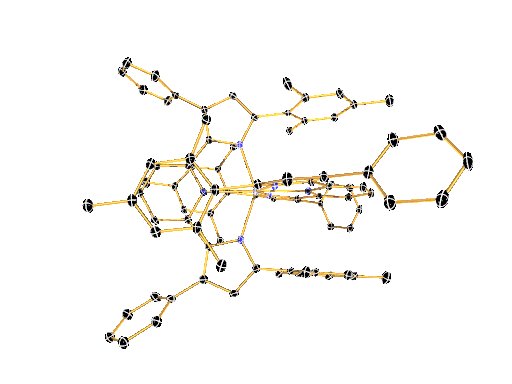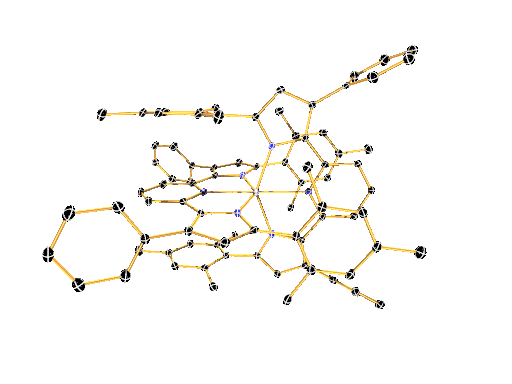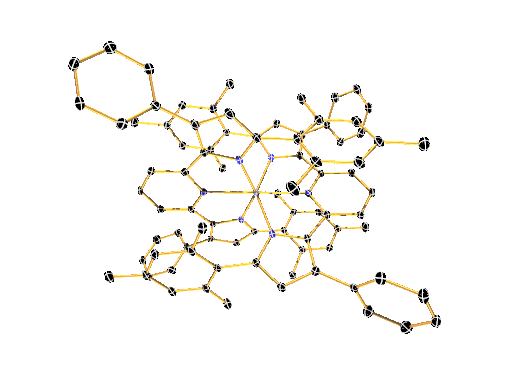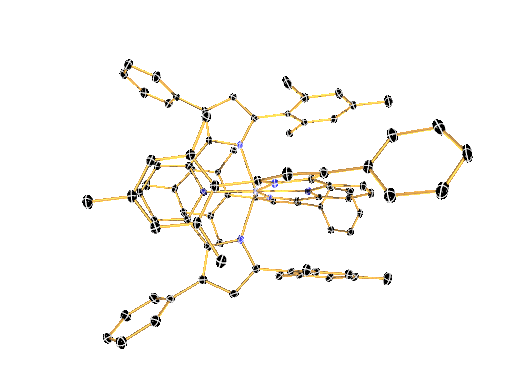
LIGAND DESIGN ENABLES CONTROL OVER PHYSICAL AND CHEMICAL PROPERTIES
The large toolbox of inorganic and organic synthesis allows precise control over the molecular structure, physical properties, and chemical reactivity of the molecules envisioned and prepared in the Milsmann Lab. We combine our expertise in synthetic chemistry with detailed physical-inorganic studies to achieve our ultimate goal to provide tailor-made materials for a wide variety of photochemical applications.

DETAILED PHOTOPHYSICAL INSIGHT INTO DELAYED FLUORESCENCE (TADF)
In collaboration with the Castellano group at North Carolina State University, we continue to study the excited state dynamics and photoluminescence mechanisms operational in our novel complexes. Interestingly, the dominant emission pathway for our luminescent group 4 transition metal and group 14 main group compounds is thermally activated delayed fluorescence rather than phosphorescence typically encountered in transition metal chromophores.

