THE MILSMANN LAB
Inorganic Chemistry Research at the University of Delaware

We have moved!
As of August 2024, the Milsmann Lab has a new home in the Department of Chemistry & Biochemistry at the University of Delaware.
RESEARCH AREAS
Research in the Milsmann lab combines the areas of physical inorganic chemistry, synthetic inorganic chemistry, and catalysis to find new solutions towards more sustainable and green chemistry. We try to utilize compounds based on earth-abundant elements in (photo)chemical processes that are traditionally dominated by precious metal catalysts. All projects in the group involve the synthesis and manipulation of air-sensitive materials under rigorously inert conditions and take advantage of the large tool box of available physical methods for in-depths analysis and characterization.



RECENT PUBLICATIONS
Below you will find a selection of our team’s most recently published papers. Click on the title or picture for a link to the article. A complete list of the group's research articles can be found here.
Anitha S. Gowda, Marisa N. Tordella, Glenn P. A. Yap, Jeffrey L. Petersen, and Carsten Milsmann
Inorganic Chemistry 2025, 64, 20949.
A series of organotetrel compounds (Si, Ge, Sn) containing pyridine dipyrrolide (PDP) ligands are reported and show intense photoluminescence resulting from a combination of prompt fluorescence and thermally activated delayed fluorescence (TADF). Kinetic analysis of the TADF emission supports increased intersystem crossing and reverse intersystem crossing rate constants for bis-PDP compared to mono-PDP compounds, highlighting the importance of symmetry-breaking charge transfer in accessing long-lived triplet excited states.
Matthew J. Goodwin, Quentin R. Loague, Marisa N. Tordella, Dylan C. Leary, Pierpaolo Vecchi, Gage N. Jackson, Carsten Milsmann, and Gerald J. Meyer
Journal of the American Chemical Society 2025, 147, 36393.
Electroabsorption spectra of three Zr(IV) photosensitizers bearing two tridentate pyridinedipyrrolide ligands, Zr(PDP)2, revealed significant changes in the polarizability between the ground and excited state without a dipole moment change. The spectral data indicates that upon the absorption of a single photon a quadrupolar excited state is formed via a simultaneous and equal charge transfer from both tridentate ligands to the Zr metal center. These data provide the first direct experimental evidence for such behavior in transition metal complexes with charge transfer excited states.
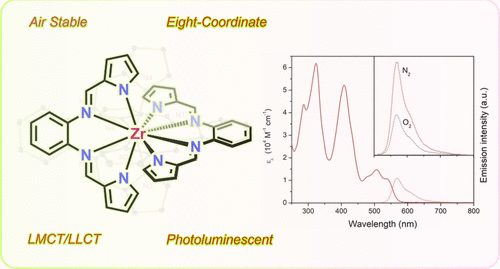
Yu Zhang, Tia S. Lee, Jeffrey L. Petersen, and Carsten Milsmann
Inorganic Chemistry 2024, 63, 9002
The eight-coordinate zirconium complex Zr(bppda)2 exhibits photoluminescence through a combination of prompt fluorescence and thermally activated delayed fluorescence (TADF) at room temperature. The lowest energy singlet and triplet excited states contain significant ligand-to-metal charge transfer character involving the d0 metal ion. Femtosecond transient absorption spectroscopy revealed slower intersystem crossing rates compared to related Zr(PDP)2 complexes, which were correlated with subtle changes in the electronic structures of the two compound classes.
Department of Chemistry & Biochemistry
University of Delaware
163 The Green
237 Brown Laboratory
Newark, DE 19716